Table of Contents
Updated
Hope this blog post helps you when you see the chemistry of computing errors. g.Subtract the accepted value from the experimental value. Divide this answer by the accepted meaning. Multiply this answer by 100 and add a% symbol that represents the answer as a percentage.
g.Subtract the accepted value from the experimental value. Divide this key by the accepted value. Multiply the majority of the answer by 100 and add the% symbol to express the answer as a percentage.
g.refusal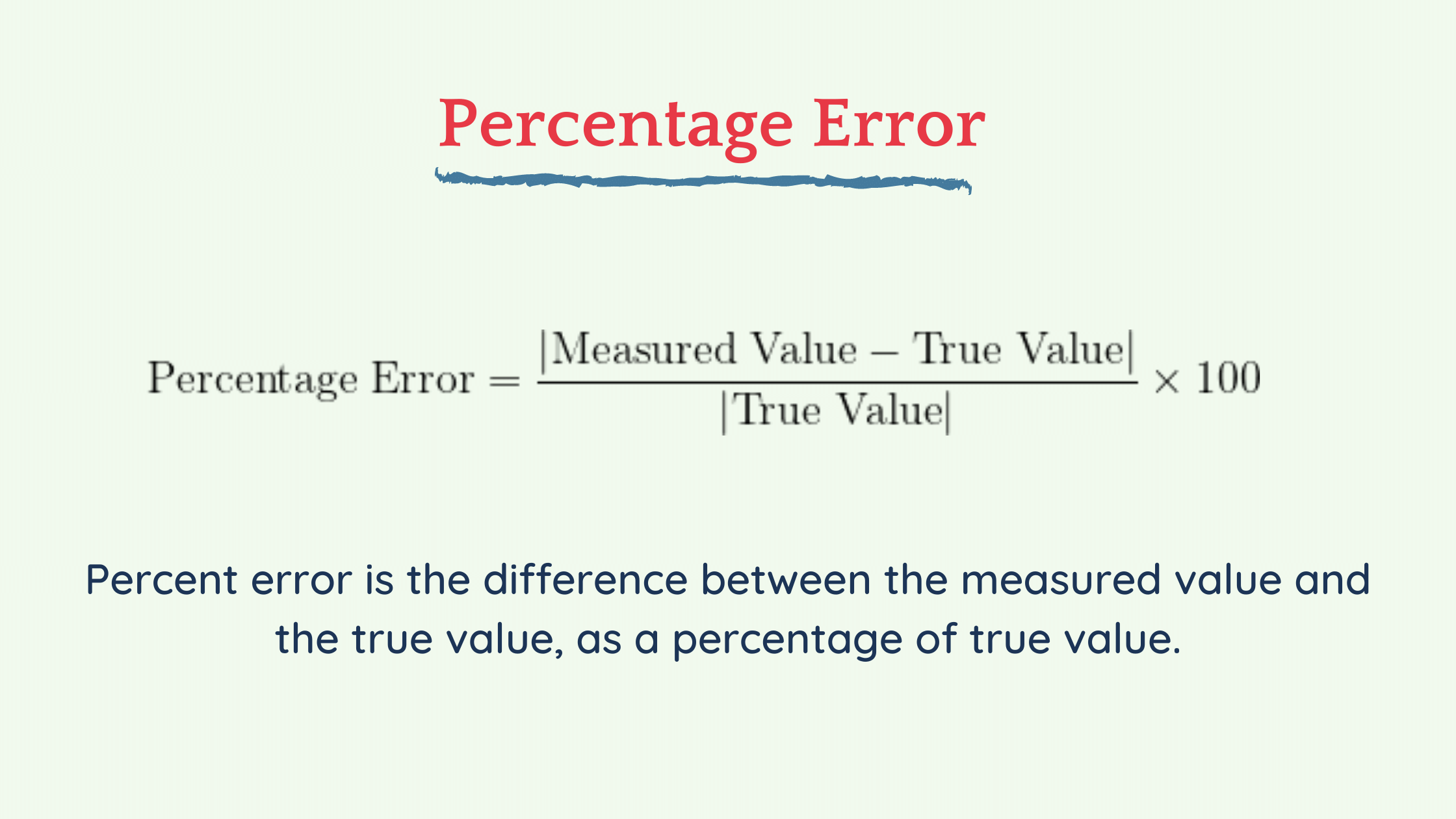
Percentages and / or percentages of errors are expressed in numerical form, the difference between an approximate or quantitative value and an exact or known win. In applied science, it is customary to indicate the difference between a measured value, as a variant experimental , and a true or specific value. How to calculate the percentage of error using a calculation example.
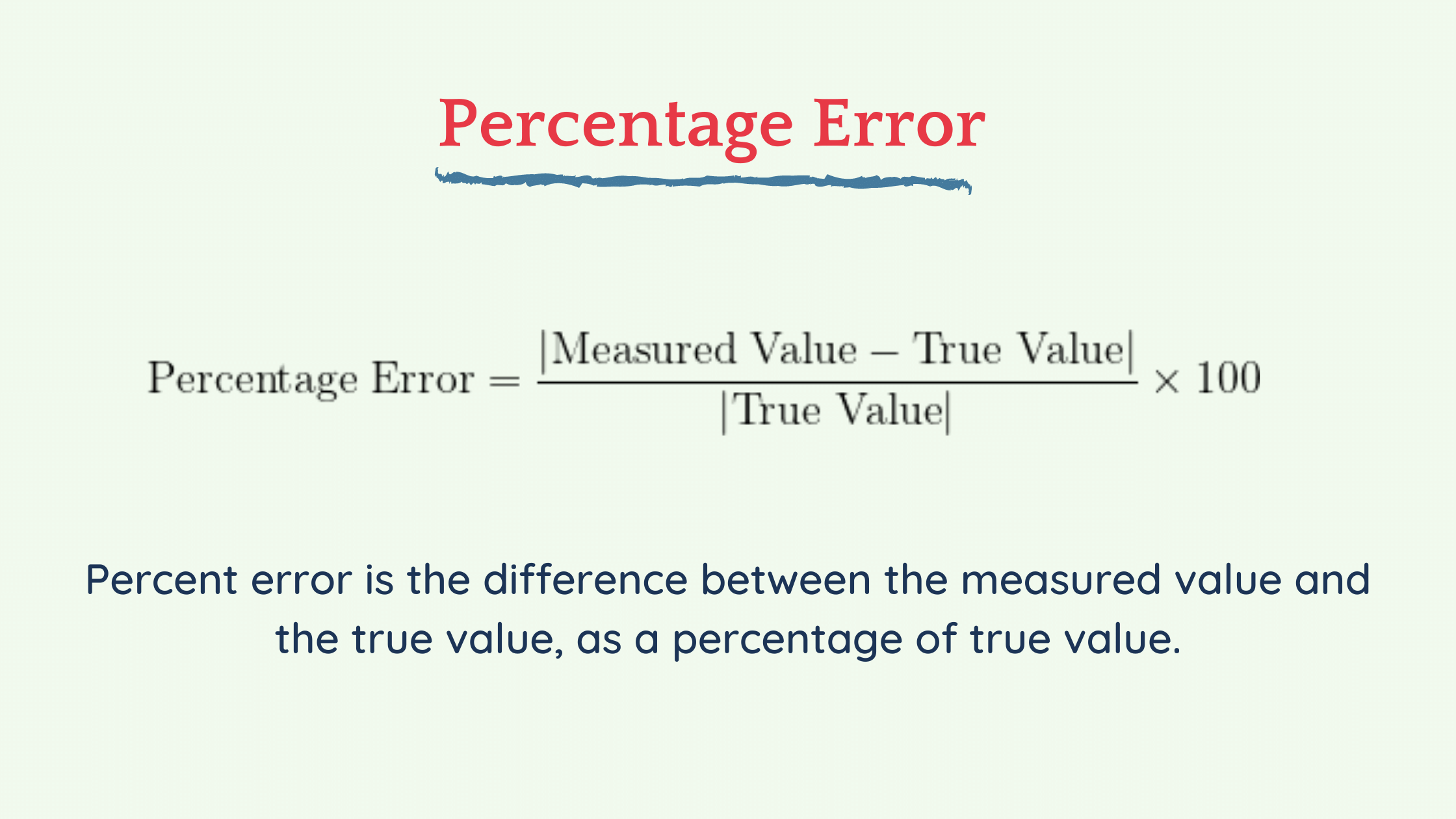
Formula For Percent Error
How do you calculate error?
Percentage error is defined as the difference between the exact pleasure and the estimated value of a given amount, divided by the exact market value, and then multiplied by 100 to actually represent it as a percentage of the exact value. The percentage of errors is | Approximate value – Exact value | / Understood exactly * 100.
Percentage error is the large difference between a measured or experimental value and an accepted or known value divided by the known value multiplied by 100%.
In some applications, percentage error is always expressed relative to a positive value. The absolute error value is divided by the agreed value and expressed as a percentage.
What is the percent error formula in chemistry?
Error percentage: the absolute value of the error, which is lost by the accepted value and is increased by 100%.
after all, in other sciences it is customary to retain a negative value in the case of materialization. It is undoubtedly important whether the error is Either positive or negative. For example, you do not expect to get a positive percentage error when comparing severe yield to theoretical yield of reaction with detergent . If a positive value was calculated, it would indicate possible problems with the procedure or unexplained reactions.
How do u calculate percent error?
The error rate is expressed as a ratio and is calculated by dividing the total number of words read by the total number of errors made. The ratio can be expressed as 1:20. That is, for every mistake, the little girl read 20 words correctly.
When checking the sign of the error, the calculation was always the experimental or measured value minus the known or theoretical value, presumably divided by the theoretical value and multiplied by 100%.
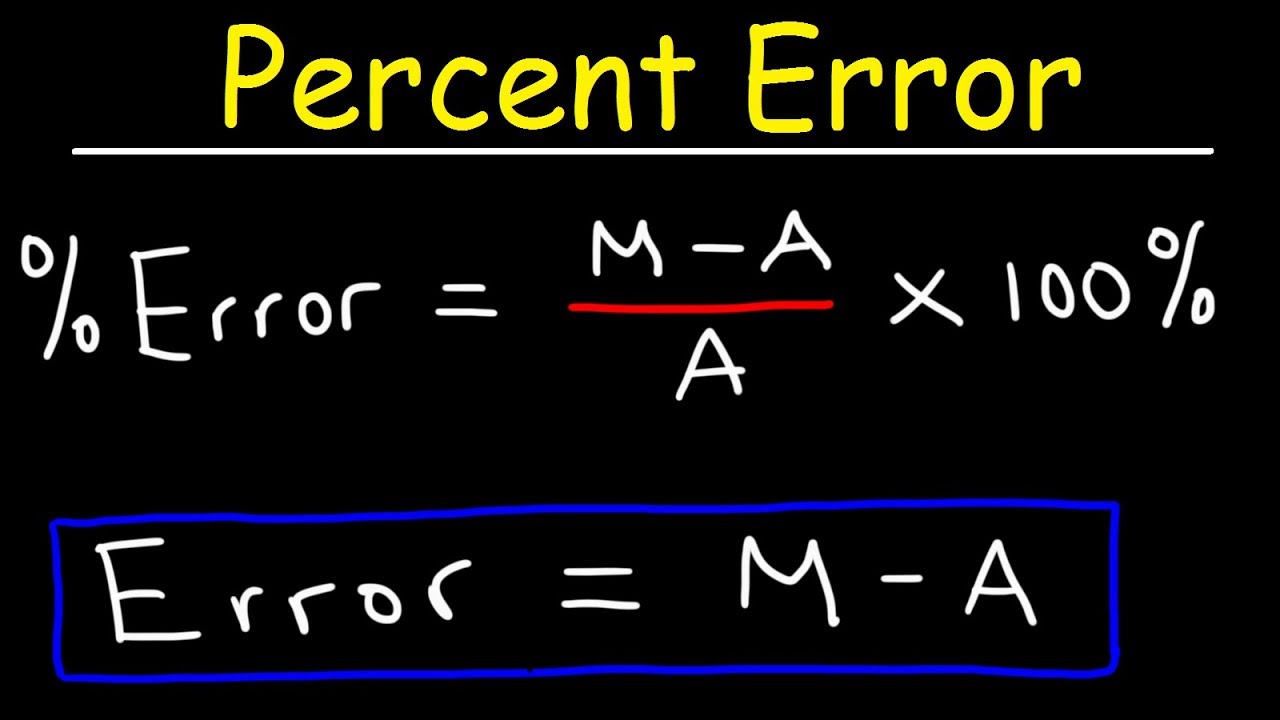
percentage error implies [experimental – theoretical] value and theoretical value x 100%.
Steps To Calculate Error Rate
- Subtract one value from another. The order of the signs does not matter as long as you omit the sign (with a specific absolute value. Subtract the theoretical value of a from the experimental value if left negative. This value is its own “error”.
- Divide the error by the exact value, or just the value of the object (not your experimental or sasea-recognized value). This will give a decimal number.
- Convert the corresponding decimal number to a percentage, improving it by 100.
- Add a percentage or% symbol to indicate an invalid selection percentage.
Example Of Calculating The Percentage Of Errors
Aluminum is prohibited in any laboratory. They measure the dimensions associated with the block and its volume in each container with a known volume of hot water. According to their calculations, the density of the aluminum road is 2.68 g / cm 3 . You can see the density of a piece of aluminum at room temperature and notice that it is 2.70 g / cm 3 . Calculate the percentage error of your measurement.
- Subtract one share from the other:
2.68-2.70 equals -0.02 - Any negative sign can be ignored if necessary (use one value): 0.02
This is an error. - Just divide the error by the true value: 0.02 / 2.70 = 0.0074074
- Multiply this value by 100% to get the percentage error:
0.0074074 y 100% = 0.74% (expressed as significant numbers ).
Significant numbers are important in science. If you informif there are too few or too few answers, this may be considered incorrect, even if you have clearly formulated the problem correctly.
Percentage Of Error Versus Absolute And Relative Error
Percentage error refers to absolute and relative error . The difference between the experimental value and the highly estimated value is absolute error. If you divide this number by a known amount, you get relative error . The percentage error is the relative error multiplied by 100%. In most cases, you use values to indicate the appropriate choice of significant digits.
Sources
- Bennett, Jeffrey; Briggs, William (2005), Using and Understanding Mathematics: A Quantitative Approach to Reasoning (3rd ed.), Boston: Pearson.
- Tornquist, Leo; Pentti; vartia, Vartia, Yrjö (1985), “How Should Relative Change Be Measured?” American Statistician, 39 (1): 43-46.
Important Points: Error Rate
- The purpose of calculating percentage error is to give a practical idea of how closevalue to your true value.
- The Percent Error is the main difference between the experimental value and the theoretical estimate, divided by the theoretical value, multiplied by 100 to get the percentage.
- In some places, the percentage error is always expressed as a solid positive number. In other cases, it is appropriate to have a positive or dangerous meaning. The sign can be used to determine if the recorded values are consistently above or below the expected values.
- Percentage error is the only type of error calculation. Absolute error and uncle’s error are two other common calculations. The error percentage is part of the final error analysis.
- Percentage error buttons are easy to spot if you omit a sign (positive or negative) in the calculation and a number with the correct number of large digits.
Updated
Are you tired of your computer running slow? Annoyed by frustrating error messages? ASR Pro is the solution for you! Our recommended tool will quickly diagnose and repair Windows issues while dramatically increasing system performance. So don't wait any longer, download ASR Pro today!

Olika Sätt Att åtgärda Kemiberäkningsfel
Verschillende Manieren Om Een scheikundige Berekeningsfout Op Te Lossen
Verschiedene Möglichkeiten Zur Behebung Von Chemieberechnungsfehlern
Różne Sposoby Naprawienia Błędu Obliczeń Chemicznych
Diverses Façons De Corriger L’erreur De Calcul De Chimie
Varias Formas De Corregir El Error De Cálculo De Química
Vari Modi Per Correggere L’errore Di Calcolo Della Chimica
Различные способы исправить ошибку расчета химии
Várias Maneiras De Corrigir O Erro De Cálculo De Química
화학 계산 오류를 수정하는 다양한 방법